Outcomes-Based Agreements in Canada: An Overview
April 22, 2021
The specialty drug industry can hardly keep up with itself.
Every month sees new miracle drugs—drugs that stop advanced cancer in patients with specific genetic profiles or that give new hope to patients born with the rarest of diseases—tumble into the market. These drugs, which require Herculean resources and ingenuity to develop, don’t come cheap. This leaves public and private decision makers with the difficult challenge of balancing timely access with budget constraints, while patients stand by.
All about access
In theory it should be simple: drug innovation saves and transforms lives, and patients deserve access to these drugs. But the assessment framework we’ve inherited from simpler drug days doesn’t have the vision and flexibility to handle the new drug development world.
As Andre Vidal-Pinheiro, head of global pricing and access at Takeda, noted in a 2020 webinar,5 “standard health technology assessment criteria are not particularly kind to orphan drugs.” By definition, these drugs serve small and highly specific patient populations, so they seldom generate the large datasets that regulators traditionally require to demonstrate clinical value. Add the high cost of these drugs to the mix and you’re left with what Vidal-Pinheiro calls “a recipe for disaster.” Manufacturers argue that these medications change lives, while regulators and payers focus on the risks and cost-benefit ratio. The result? Delayed access.
These challenges are playing themselves out in our national backyard. The tumor-agnostic oncology medication Vitrakvi, for example, received a negative recommendation from pCODR’s Expert Review Committee (pERC) because the limited clinical data on the drug left the Committee unsure of its advantage over existing therapies.17 While the manufacturer’s resubmission16 moves through the system, patients continue to wait. The recent approval of Zolgensma 5—reportedly the most expensive drug in the world at $2 million per year5—is compelling governments to think about access in new ways. At over $300,000 per year, the breakthrough cystic fibrosis drug Trikafta, currently undergoing priority review in Canada18, will likewise require innovative access solutions to thrive, and the new drug pipeline promises more of the same.
Sharing the risk
Outcomes-based agreements (OBAs), also known as value-based, managed-access, or performance-based agreements, are poised to bridge such access gaps. Based on the principle of dividing risk between manufacturers and payers, these agreements typically include both a data-collection component—to better understand real-world efficacy—and a commercial agreement to delineate the risk-sharing terms.
What is an outcomes-based agreement?8
An agreement between a manufacturer and a payer in which the manufacturer will issue a refund or rebate to the payer based on how well the therapy performs in a real-world patient population, measured against an agreed-upon, pre-defined set of benchmarks.
A number of countries with single-payer healthcare systems have developed formalized risk-sharing pathways for rare and high-cost diseases. Recognized globally as an OBA leader, Italy has had such arrangements in place since 2006.19 Several other European countries have followed suit, with OBAs that enable quick listing followed by reassessment based on insights from real-world evidence (RWE).20
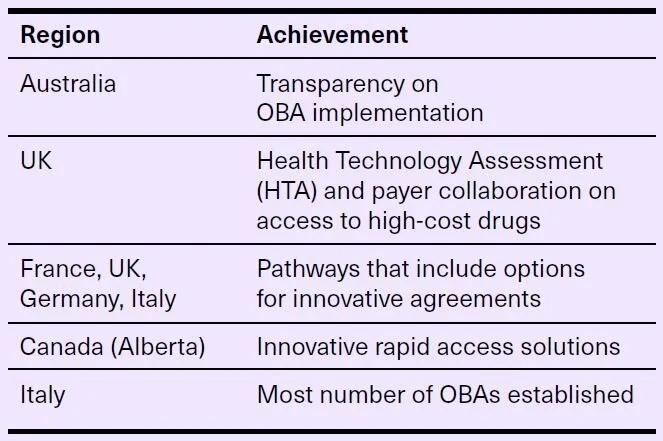
Leaders to learn from
These regions have shown leadership in the OBA realm and can serve as models for future OBA development and implementation.8The Canadian OBA landscape
While several steps behind these jurisdictions, Canada has recognized the need to take action. To help Canadians with rare diseases access the drugs they need, the country plans to invest up to $1 billion over two years into a national strategy that includes pay-for-performance and risk-pooling models21 —in other words, OBAs.
Based on the findings of a 2017 global report, Canada already had 16 publicly disclosed “innovative contracts” at the time,7 and the number has undoubtedly grown since then. While the confidential nature of OBAs has constrained the free exchange of information about these agreements, speakers in public forums have confirmed the existence of OBA-style schemes in Canada for a handful of specific drugs, such as Spinraza and Revestive.5,8
An OBA by another name - Spinraza's story
In a virtual conference organized by the Canadian Organization for Rare Diseases (CORD), Quebec-based SMA patient advocate Catherine Boivin alluded to “a type of managed access approval” for Spinraza,5 suggesting an OBA-style agreement. What steps might have led to this development?
It all began in December 2018, when Quebec’s INESSS announced its recommendation to list spinal muscular atrophy (SMA) medication Spinraza on the “promise of therapeutic value,” with the possibility of delisting it in the future if real-world evidence shows insufficient benefit.22 Shortly thereafter, an update to the Saskatchewan formulary explained that patients seeking continued coverage for Spinraza “will be required to undergo ongoing assessment to monitor for improvement over time and must meet renewal criteria for continuation of treatment.”23 Ontario soon joined Quebec and Saskatchewan in broadening criteria for Spinraza access to include adults “on a case-by-case basis.”24 London, Ont. pediatric neurologist Dr. Craig Campbell applauded the agreement, stating it would allow the SMA community to “collectively document the long-term real-world data to further demonstrate the positive impact of [Spinraza.]”24 It seems these provinces parlayed pCPA [pan-Canadian Pharmaceutical Alliance] negotiations25 into an agreement about how to pay for Spinraza—an agreement that looks a lot like an OBA.
When the shoe fits
Listing decisions depend on a web of interconnected factors, which in the case of novel medications include some gaping unknowns. This may help explain why only 20% of medications assessed by the UK’s National Institute for Health and Care Excellence (NICE) received a final recommendation after a single committee meeting.26 A review of NICE’s assessment process attributed this sluggish performance to “mischaracterization of a technology’s value and effectiveness by manufacturers, leading to uncertainty of doubt surrounding its cost-effectiveness following independent academic review.”26 The good news: in the majority of cases, “decision optimization” involving enhanced commercial agreements [read: OBAs] removed the barriers to a positive decision.26
This is not to say that OBAs make sense for all specialty drugs. In fact, as advised by the UK’s 2021 Commercial Framework for New Medicines, complex arrangements such as OBAs should “only be considered once simple discounts (which facilitate fastest access) have been demonstrated to be unsuitable.”27
OBAs offer the greatest benefit in the following circumstances:28
- Variable response: When clinical trials suggest that only a limited proportion of patients (e.g. 50%) reach a desired health outcome, OBAs can reduce a payer’s risk by limiting ongoing reimbursement to patients who meet agreed-upon outcome criteria.
- Limited data: When promising but incomplete early clinical trial data makes it difficult to assess a drug’s performance, an OBA can provide access to patients with no treatment alternatives to meet their needs.
- Stalled negotiations: When the drug manufacturer and the HTA and/or payer disagree on the magnitude of therapeutic benefit suggested by clinical trial data, an OBA can help patients access treatment earlier, with continued access dependent on proof of benefit.
From intention to implementation
Manufacturers and payers who cut their teeth on simple financial contracts may wonder how to get the OBA ball rolling—and with good reason. As market access expert Dr. Philip Spearpoint asserted in a talk on managed entry agreements in the EU, “if you think agreeing to an OBA is difficult, wait until you get to the implementation.”29 The biggest challenge? Devising a framework that guides all stakeholders toward an achievable, mutually satisfactory agreement.
Fortunately, future-oriented stakeholders are working on strategies to enhance the opportunity for OBA implementation in Canada. Alberta’s Institute for Health Economics is creating tools and resources to support risk-management agreements and other innovative funding options.30 The Canadian Centre for Applied Research in Cancer Control is developing a framework for using RWE to support oncology drug funding decisions, with support from the Canadian Institute for Health Research under the CanREValue initiative.31 And for the RWE & OBA Working Group, co-creating implementation solutions with Canada’s OBA stakeholders tops the priority list.
From the manufacturer perspective, the success of an OBA rests on good planning—ideally with a cross-functional OBA team to take the lead on negotiation and implementation. After confirming a drug’s suitability for an OBA, the team must ensure that both parties at the negotiating table—manufacturer and payer—agree on the outcomes of interest and how to measure them.28 To avoid unpleasant surprises down the line, the negotiated agreement should cover all aspects of the OBA—from patient consent and follow-up to data collection, reporting and adjudication.
To close the loop, manufacturers can share their OBA learnings with the industry, as did Pfizer in a 2017 public document describing the reporting requirements and pricing conditions of its Australian managed entry agreement for the lung cancer drug Xalkori.32 Such transparent sharing, which need not involve disclosure of confidential information, helps move the OBA space forward for all players.
There is a lot to celebrate at this juncture: OBAs have entered the public conversation, implementation frameworks are on the design table, and early adopters are making their mark. The industry now needs to move forward with customized OBAs that reflect the specific challenges of each drug, delineation of roles and responsibilities, as well as mechanisms to share OBA practices so the space can mature.
A fuller integration of OBAs into the specialty drug ecosystem will require creativity, collaboration and courage. By all indications, rare-disease and precision oncology drugs will lead the charge. Patients are counting on it.
References:
Hoskyn SL. Explaining public reimbursement delays for new medicines for Canadian patients. Innovative medicines Canada. http://innovativemedicines.ca/wp-content/uploads/2020/06/20200630-CADTH-TTL-Poster-FINAL.pdf
Health Canada approves Zolgensma, the one-time treatment for pediatric patients with SMA. Cision. Dec. 16, 2020. https://www.newswire.ca/news-releases/health-canada-approves-zolgensma-r-the-one-time-gene-therapy-for-pediatric-patients-with-spinal-muscular-atrophy-sma-1-886604228.html
Alberta Zolgensma listing announcement. https://www.youtube.com/watch?v=BuHRfRZo9Wo&feature=emb_logo
Pipeline Monitor 2020. Patented Medicine Prices Review Board. https://www.canada.ca/content/dam/pmprb-cepmb/documents/npduis/analytical-studies/meds-pipeline-monitor/2020/MPM-2020-en.pdf
Designing the blueprint for pan-Canadian rare drug program. CORD virtual conference. Dec. 16, 2020. https://www.youtube.com/watch?v=OggDgpzqFBY
More than half of all health plans use outcomes-based contracts. Avalere press release. Oct. 1, 2019. https://avalere.com/press-releases/more-than-half-of-health-plans-use-outcomes-based-contracts
Innovative pharma contracts: when do value-based arrangements work? McKinsey & Company. Oct. 19, 2017. https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/innovative-pharma-contracts-when-do-value-based-arrangements-work
20Sense original research.
Clinical review report: Nusinersen (Spinraza). CADTH. January 2018. https://www.ncbi.nlm.nih.gov/books/NBK533991/#cl1.s1.
Successful market access for gene therapies—strategic challenges and possible solutions. SKC Beratungsgesellschaft mbH 2020. https://skc-beratung.de/whitepaper/GentherapieWhitepaper_ENG.pdf
Families of Alberta children suffering from spinal muscular atrophy (SMA) may now be eligible to receive funding for gene replacement therapy treatment. Cure SMA news release. https://curesma.ca/2021/01/28/zolgensma/
It’s time to get loud for Canadians with cystic fibrosis. CF Get Loud. https://www.cfgetloud.ca/
Life-saving drugs FAQs. Cystic Fibrosis Trust. https://www.cysticfibrosis.org.uk/the-work-we-do/campaigning-hard/life-saving-drugs/life-saving-drugs-faqs
Georgieva K. Cystic Fibrosis Canada says “life-changing” drug coming to Canada, but approval months away. CBC News. Nov. 11, 2020. https://www.cbc.ca/news/canada/windsor/cystic-fibrosis-community-rejoicing-trikafta-1.5798421#:~:text=Trikafta%20costs%20roughly%20%24300%2C000%20US%20a%20year
Melamed D. Trikafta soon to be up for approval, Cystic Fibrosis Canada reporting. Cystic Fibrosis News Today. Nov. 13, 2020. https://cysticfibrosisnewstoday.com/2020/11/13/trikafta-expected-soon-up-for-health-canada-approval-cyctic-fibrosis-canada-says/
pCODR Expert Review Committee final recommendation for larotrectinib. https://cadth.ca/sites/default/files/pcodr/Reviews2019/10159LarotrectinibNTRK%2BSolidTumours_fnRec_REDACT_31Oct201_ChairApproved_final.pdf
CADTH reimbursement review: Larotrectinib. https://www.cadth.ca/larotrectinib
Cystic Fibrosis Canada. Trikafta. https://www.cysticfibrosis.ca/our-programs/advocacy/access-to-medicines/trikafta
Upton J. Risk sharing, Italian style. PharmaExec.com. March 19, 2018. https://www.pharmexec.com/view/risk-sharing-italian-style
Drawing the blueprint for Canada’s rare drug program 2022. CORD webinar. January 29, 2021. https://www.youtube.com/watch?v=cBD5hsD7q8Y&feature=emb_logo
Building a national strategy for high-cost drugs for rare diseases online engagement. https://www.canada.ca/en/health-canada/programs/consultation-national-strategy-high-cost-drugs-rare-diseases-online-engagement.html
Santé et services sociaux Québec. Communiqué, Dec. 18, 2018. https://www.msss.gouv.qc.ca/ministere/salle-de-presse/communique-1715/
Saskatchewan formulary bulletin: update to the 62nd edition of the Saskatchewan formulary. May 1, 2019. https://formulary.drugplan.ehealthsask.ca/Bulletins/Bulletin-0176-May-2019.pdf
Ontario grants broader access to SPINRAZA™ (nusinersen) for patients living with spinal muscular atrophy (SMA). Newswire Canada. June 13, 2019. https://www.newswire.ca/news-releases/ontario-grants-broader-access-to-spinraza-tm-nusinersen-for-patients-living-with-spinal-muscular-atrophy-sma--835281948.html
Santé et services sociaux Québec. Amyotrophie spinale 5q – La ministre McCann annonce que les personnes atteintes des types II et III de la maladie auront accès au médicament Spinraza. https://www.msss.gouv.qc.ca/ministere/salle-de-presse/communique-1715/
Walton MJ et al. A review of issues affecting the efficiency of decision making in the NICE single technology appraisal process. Pharmacoeconom 2019;3:403.
NHS commercial framework for new medicines. https://www.england.nhs.uk/wp-content/uploads/2021/02/B0255-nhs-commercial-framework-for-new-medicines.pdf
Real-world evidence and outcomes-based agreements working group. 2019 research & outputs. https://static1.squarespace.com/static/58fd16af1b631b1afffae9e0/t/5f8e437dd9a86a5bd6b09076/1603158910148/2019_Nov_RWE_OBA_WorkingGroup_ExecSummary.pdf
Spearpoint P. Implementing a managed entry agreement within the EU. NextLevel Pharma presentation. Oct. 13, 2015. https://www.youtube.com/watch?v=wSEDxa6MOBc&feature=youtu.be
Health Technology Innovation Platform. Institute of Health Economics. https://www.ihe.ca/research-programs/innovation/htip
CanREValue: value-based decisions from real-world evidence. Canadian Centre for Applied Research in Cancer Control. https://cc-arcc.ca/canrevalue/
Public summary document. March 2017 PBAC meeting. Section 6.09, crizotinib. https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2017-03/files/crizotinib-psd-march-2017.pdf